Nomenclature worksheet 6 binary covalent compounds – Embark on a journey of discovery with Nomenclature Worksheet 6: Binary Covalent Compounds, a comprehensive guide that unravels the intricacies of naming these essential chemical entities. Delve into the systematic approach of naming binary covalent compounds, exploring their unique characteristics and diverse applications in our daily lives.
Through a series of engaging examples and interactive exercises, this worksheet empowers learners to master the art of nomenclature, equipping them with a solid foundation for further exploration in the realm of chemistry.
Binary Covalent Compounds Nomenclature
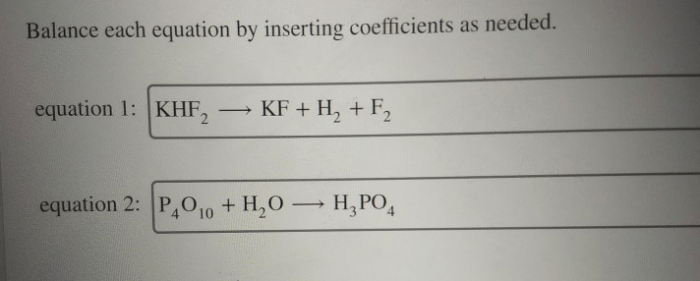
Binary covalent compounds are compounds formed between two non-metallic elements. They are named using the following rules:
- The first element in the name is the one that comes first in the periodic table.
- The second element in the name is the one that comes second in the periodic table.
- The suffix -ide is added to the root of the second element’s name.
For example, the binary covalent compound formed between hydrogen and chlorine is called hydrogen chloride.
Types of Binary Covalent Compounds
There are two main types of binary covalent compounds:
- Polar covalent compoundsare compounds in which the electrons are not shared equally between the two atoms. This results in a partial positive charge on one atom and a partial negative charge on the other atom.
- Nonpolar covalent compoundsare compounds in which the electrons are shared equally between the two atoms. This results in no partial charges on either atom.
The polarity of a binary covalent compound can be determined by the difference in electronegativity between the two atoms. Electronegativity is a measure of an atom’s ability to attract electrons. The greater the difference in electronegativity between two atoms, the more polar the bond between them will be.
Properties of Binary Covalent Compounds
Binary covalent compounds have a variety of physical and chemical properties. Some of the most common properties include:
- Low melting and boiling points: Binary covalent compounds typically have low melting and boiling points because the forces between the molecules are relatively weak.
- Solubility: Binary covalent compounds are generally soluble in organic solvents, such as alcohol and ether.
- Reactivity: Binary covalent compounds can be reactive, especially if they contain polar bonds.
The properties of binary covalent compounds are related to their structure. The type of bond between the atoms, the polarity of the bond, and the shape of the molecule all affect the properties of the compound.
Naming Binary Covalent Compounds Worksheet, Nomenclature worksheet 6 binary covalent compounds
The following worksheet can be used to practice naming binary covalent compounds:
- Instructions: Name the following binary covalent compounds:
- HCl
- NH3
- CO 2
- CH 4
- H 2O
Answers:
- HCl: hydrogen chloride
- NH 3: ammonia
- CO 2: carbon dioxide
- CH 4: methane
- H 2O: water
Applications of Binary Covalent Compounds
Binary covalent compounds are used in a wide variety of applications. Some of the most common applications include:
- As solvents: Binary covalent compounds, such as alcohol and ether, are used as solvents to dissolve other substances.
- As fuels: Binary covalent compounds, such as methane and propane, are used as fuels to generate heat and energy.
- As building materials: Binary covalent compounds, such as silicon dioxide, are used as building materials to make glass and ceramics.
Binary covalent compounds are essential to our everyday lives. They are used in a wide variety of products, from the food we eat to the clothes we wear.
FAQ Resource: Nomenclature Worksheet 6 Binary Covalent Compounds
What is the primary focus of Nomenclature Worksheet 6?
Nomenclature Worksheet 6 focuses on providing a comprehensive understanding of the rules and conventions involved in naming binary covalent compounds.
How does the worksheet facilitate learning?
The worksheet employs a combination of clear explanations, illustrative examples, and interactive exercises to enhance comprehension and retention.
What are the benefits of completing this worksheet?
Completing this worksheet empowers learners with the ability to accurately name binary covalent compounds, a crucial skill for further studies in chemistry.